About IGF-1R
IGF-1R is a transmembrane receptor tyrosine kinase that plays a central role in regulating cell growth, proliferation, differentiation, and survival, and is involved in both normal development and disease pathophysiology.1, 2 IGF-1R is primarily activated by its high-affinity ligands, IGF-1 and IGF-2, though it can also bind insulin with lower affinity.1, 2
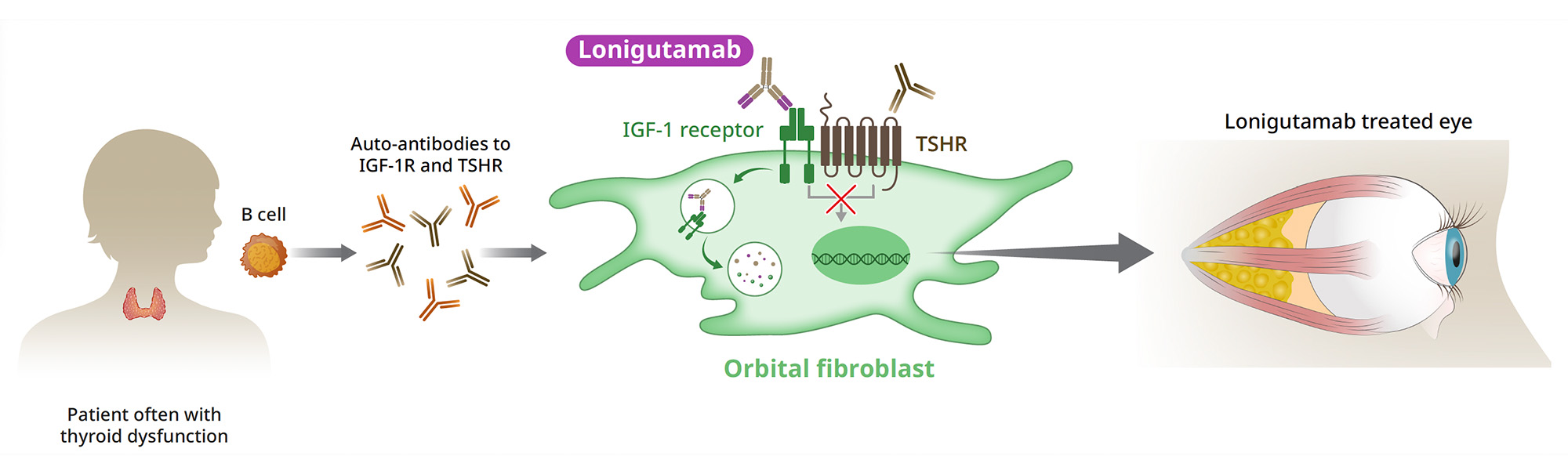
IGF-1R and Thyroid Eye Disease
IGF-1R plays a significant role in the pathogenesis of TED.2-4 It forms a functional receptor complex along with thyroid-stimulating hormone receptor (TSHR) on the surface of orbital fibroblasts behind the eye.5 Evidence indicates that patients with TED produce autoantibodies that stimulate this receptor complex, causing hyperactivation of orbital fibroblasts and immune cells which mediate the disease process in susceptible individuals.6 Inhibiting IGF-1R with teprotumumab, a mAb, has been shown to reduce the characteristic eye bulging (proptosis) and other serious clinical manifestations in patients with TED.7, 8
References
1 LeRoith, D., Holly, J. M. P. & Forbes, B. E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 52, 101245 (2021).
2 Osher, E. & Macaulay, V. M. Therapeutic Targeting of the IGF Axis. Cells 8, 895 (2019).
3 Smith, T. J., Hegedüs, L. & Douglas, R. S. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves’ orbitopathy. Best Pr. Res. Clin. Endocrinol. Metab. 26, 291–302 (2012).
4 Ugradar, S. et al. Teprotumumab for non-inflammatory thyroid eye disease (TED): evidence for increased IGF-1R expression. Eye 35, 2607–2612 (2021).
5 Krieger, C. C., Neumann, S., Place, R. F., Marcus-Samuels, B. & Gershengorn, M. C. Bidirectional TSH and IGF-1 Receptor Cross Talk Mediates Stimulation of Hyaluronan Secretion by Graves’ Disease Immunoglobins. J. Clin. Endocrinol. Metab. 100, 1071–1077 (2015).
6 Smith, T. J. & Janssen, J. A. M. J. L. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocr. Rev. 40, 236–267 (2018).
7 Douglas, R. S. et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N. Engl. J. Med. 382, 341–352 (2020).
8 FDA Drug Approval Package: Tepezza (teprotumumab-trbw) https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761143s000lbl.pdf
