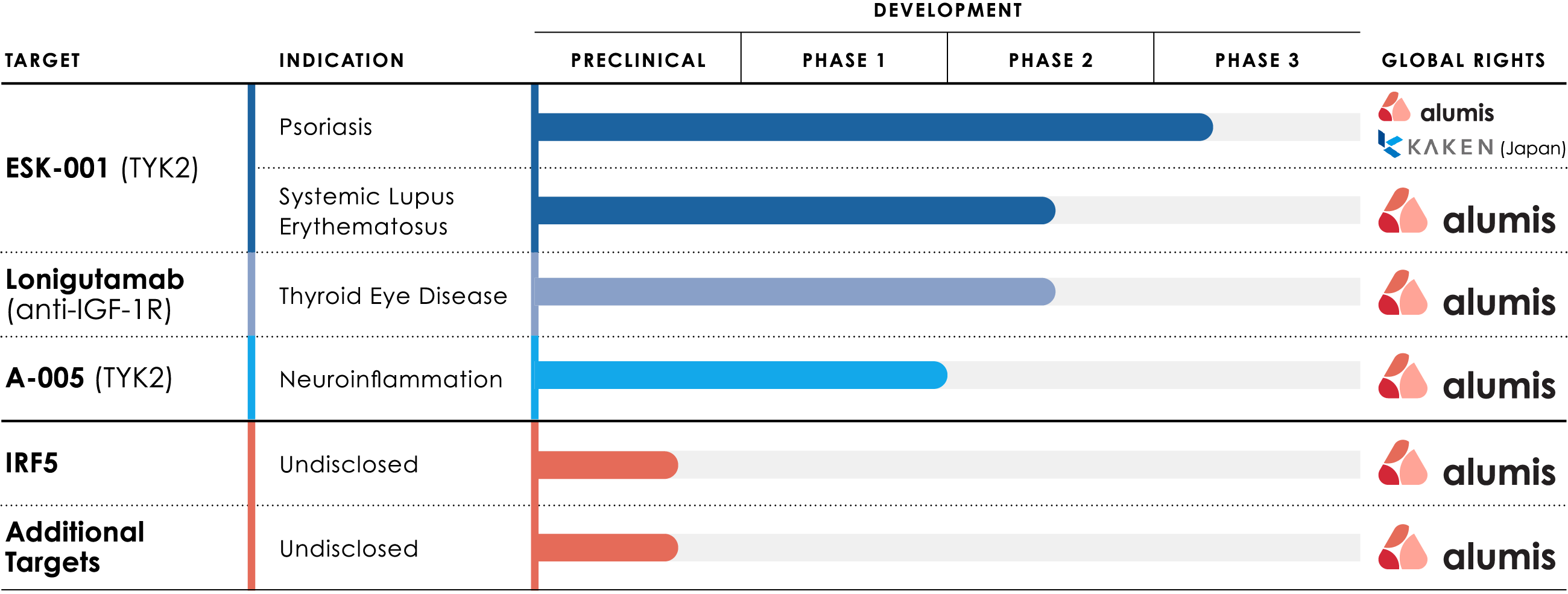
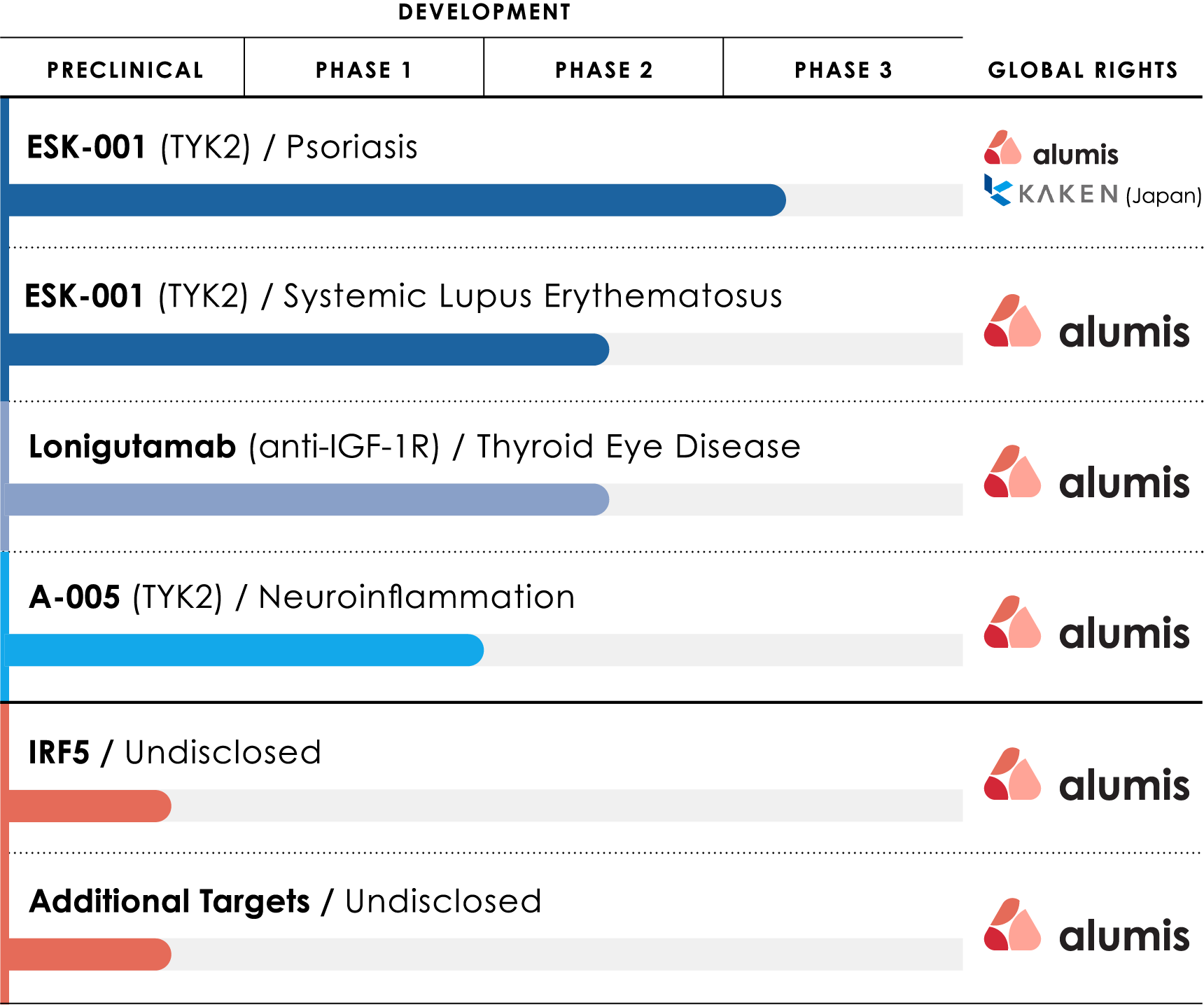
Our TYK2 franchise is led by ESK-001, a TYK2 inhibitor currently in clinical development for moderate to severe plaque psoriasis and systemic lupus erythematosus (SLE).
This highly selective inhibitor has the potential to effectively treat multiple immune-mediated indications.
We are also developing A-005, a CNS-penetrating, allosteric TYK2 inhibitor for neuro-inflammatory diseases such as multiple sclerosis (MS).
Beyond TYK2, we have several earlier-stage discovery assets, comprised of targets identified by our precision data analytics platform, for the potential treatment of immune-mediated indications.
About ESK-001
An oral, next-generation TYK2 inhibitor
Our lead candidate ESK-001 is an investigational oral, highly selective, next-generation tyrosine kinase 2 (TYK2) inhibitor with a differentiated pharmacological profile compared with other available or investigational molecules with similar mechanism of action. ESK-001 is being evaluated in Phase 3 clinical trials for the treatment of moderate to severe plaque psoriasis and a Phase 2b clinical trial for the treatment of systemic lupus erythematosus (SLE).
About A-005
A potential first-in-class CNS-penetrant allosteric TYK2 inhibitor
A-005 is designed to achieve maximal TYK2 inhibition and to cross the blood brain barrier for localized treatment both within the CNS and in the periphery, supporting its potential across multiple TYK2-mediated indications. TYK2 is a protein that plays a role in mediating signaling responses to key proinflammatory cytokines, including interleukin (IL)-23 and type 1 interferon.
A-005 completed a Phase 1 clinical trial in healthy participants and is expected to enter a Phase 2 clinical trial in patients with multiple sclerosis in the second half of 2025.
Additional Programs / Discovery Efforts
Beyond TYK2, we have several earlier-stage discovery assets comprised of targets identified by our precision data analytics platform for the potential treatment of immune-mediated indications.
Publications
Efficacy and Safety of ESK-001, a Highly Selective Oral TYK2 inhibitor, in Moderate-to-Severe Plaque Psoriasis: Long-term Phase 2 Results
American Academy of Dermatology (AAD) Annual Meeting
March 8, 2025
Patient-Reported Outcomes in the Phase 2 Studies of ESK-001, an Oral Allosteric TYK2 Inhibitor, in Adults with Moderate-to-Severe Plaque Psoriasis
American Academy of Dermatology (AAD) Annual Meeting
March 7, 2025
Pharmacokinetics, Safety, and Tolerability of ESK-001, an Allosteric TYK2 Inhibitor for Plaque Psoriasis: Evaluation in Asian Populations Compared to Caucasians
American Academy of Dermatology (AAD) Annual Meeting
March 7, 2025
ESK-001, an Allosteric TYK2 Inhibitor, Modulates Disease and TYK2-related Pathway Transcriptomic and Proteomic Biomarkers in Psoriasis STRIDE Trial Patients
American Academy of Dermatology (AAD) Annual Meeting
March 7, 2025
Safety, Tolerability, and Pharmacokinetics of A-005: A Selective Brain-Penetrant TYK2 Inhibitor for CNS Inflammatory Diseases in Healthy Volunteers Following Single and Multiple Ascending Doses
Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum
February 28, 2025
A-005, A Selective Oral Brain Penetrant TYK2 Inhibitor, Modulates Astrocytes and Microglia
Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum
February 28, 2025
Safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral allosteric TYK2 inhibitor ESK-001 using a randomized, double-blind, placebo-controlled study design
Clinical and Translational Science
November 27, 2024
ESK-001, an Allosteric TYK2 Inhibitor, Maximally Suppresses Type 1 Interferon, a Therapeutic Pathway Central to SLE and CLE
ACR Convergence 2024
November 18, 2024
Novel Role of TYK2 mechanism in SLE Pathogenesis via T Cell and B Cell Pathways
ACR Convergence 2024
November 17, 2024
ESK-001, An allosteric TYK2 Inhibitor, Downregulates Biomarkers of Disease and TYK2 Activity
European Academy of Dermatology & Venereology (EADV) Congress
September 27, 2024
Efficacy and Safety of ESK-001, a Highly Selective Oral TYK2 Inhibitor, in Moderate-toSevere Plaque Psoriasis: Phase 2 results through Week 28
European Academy of Dermatology & Venereology (EADV) Congress
September 27, 2024
Patient-Reported Outcomes in the Randomized, Double Blind Phase 2 Study of ESK-001, an Oral Allosteric TYK2 Inhibitor, in Adults with Moderate-to-Severe Plaque Psoriasis
European Academy of Dermatology & Venereology (EADV) Congress
September 25, 2024
Exploratory Exposure Response (E-R) Analysis of ESK-001, An allosteric oral TYK2 inhibitor, in Patients with Psoriasis
European Academy of Dermatology & Venereology (EADV) Congress
September 25, 2024
Efficacy and Safety of ESK-001, a Highly Selective Oral TYK2 Inhibitor, in a Phase 2 Study in Adults with Moderate-to-severe Plaque Psoriasis (STRIDE)
American Academy of Dermatology (AAD) Annual Meeting
March 9, 2024
Pharmacokinetic and Pharmacodynamic Characteristics of ESK-001, an Oral Allosteric TYK2 Inhibitor, in Phase 1 Healthy Volunteer Trials
American Academy of Dermatology (AAD) Annual Meeting
March 9, 2024
A Selective, Allosteric TYK2 Small-molecule Inhibitor Modulates Immune Cell Functions and Ameliorates Experimental Autoimmune Encephalomyelitis
Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum
March 1, 2024
